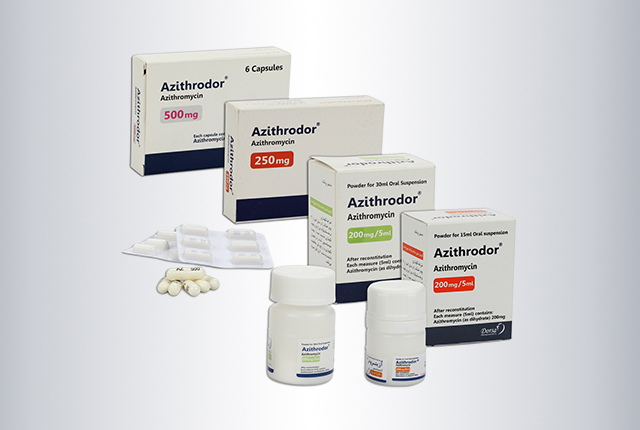
Azitrodor®
Here is a summary of the information about Azitrodor®. If there is anything you do not understand, please ask your doctor or pharmacist to explain it to you.
- Keep out of the reach of children.
- Do not use after the expiry date printed on the label.
- FDA pregnancy category: B
- Rx only.
- Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use.
Gerenal information
Patient Information
Azithromycin is one of a group of antibiotics called macrolides. It is used for the treatment of a number of bacterial infections. This includes middle ear infections, strep throat, pneumonia, traveler's diarrhea, and certain other intestinal infections.
What Azitrodor® is used for:
It is used to treat infections caused by certain bacteria and other micro-organisms, which include: chest, throat or nasal infections (such as bronchitis, pneumonia, tonsillitis, sore throat pharyngitis and sinusitis), ear infections, skin and soft tissue infections (such as an abscess or boil), sexually transmitted diseases caused by organisms called Chlamydia trachomatis and Neisseria gonorrhoea.
Tell your doctor or pharmacist before using Azitrodor® if:
- You have kidney problems, heart conditions, liver problems.
- You have myasthenia gravis (a condition that causes certain muscles to become weak)
- You are taking any ergot derivatives such as ergotamine (used to treat migraine) as these
medicines should not be taken together with Azitrodor®.
Know all the medicines you take. Keep a list of them with you to show your doctor and pharmacist.
Who should not take Azitrodor®:
If you are allergic to azithromycin or any other macrolide antibiotic such as erythromycin or
clarithromycin or any of the ingredients of this medicine. An allergic reaction may cause skin rash or wheezing.
How to take your Azitrodor®:
- Always take this medicine exactly as your doctor has told you. Check with your doctor or
pharmacist if you are not sure. The capsules should be swallowed whole.
- Azitrodor® capsules should not be taken by children weighing less than 45 kg.
- You should tell your doctor if you have kidney or liver problems as your doctor may need to alter the normal dose.
- The label on the pack will tell you which dose you should take. If you are still not sure, ask your doctor or pharmacist.
- Always continue with the course even if you feel better. If your infection gets worse or you do not start to feel better within a few days or a new infection develops, go back and see your doctor.
What to do if you forget to take a dose:
If you forget to take Azitrodor® take it as soon as you can. Take your next dose at the right time. Do not take a double dose to make up for a forgotten dose.
While taking your medicine:
- Tell your doctor immediately if you feel your heart beating in your chest or have an abnormal heartbeat, or get dizzy or faint or suffer from any muscle weakness when taking Azitrodor®.
- If you develop diarrhea or loose stools during or after treatment, tell your doctor at once. Do not take any medicine to treat your diarrhea without first checking with your doctor. If your diarrhea continues, please inform your doctor.
- You should take Azitrodor® either 1 hour before a meal or 2 hours after a meal.
- Azitrodor® is not expected to affect your ability to drive or use machines.
- You must talk to a doctor if you do not feel better or if you feel worse.
Call your doctor right away if you have:
- sudden wheeziness, difficulty in breathing, swelling of eyelids, face or lips, rash or itching
(especially affecting the whole body)
- severe or prolonged diarrhoea, which may have blood or mucus in it, during or after treatment
with Zithromax as this may be a sign of serious bowel inflammation
- severe skin rash causing redness and flaking
- rapid or irregular heartbeat
- low blood pressure
- Serious skin reactions: blistering of the skin, mouth, eyes, and genitals (Stevens-Johnson Syndrome (SJS)), blistering of the skin, severe skin reaction (Toxic Epidermal Necrosis (TEN))
, skin rash accompanied by other symptoms such as fever, swollen glands, and an increase
of eosinophils (a type of white blood cell). A rash appears as small, itchy red bumps (Drug
Reaction with Eosinophilia and Systemic Symptoms (DRESS)), skin eruption that is characterised by the rapid appearance of areas of red skin studded with small pustules (small blisters filled with white/yellow fluid) (Acute Generalized Exanthematous Pustulosis (AGEP)).
Stop taking azithromycin if you develop these skin symptoms and contact your doctor or seek
medical attention immediately.
What to do if you take too many Azitrodor®:
If you take too much Azitrodor® you may feel unwell. Tell your doctor or contact your nearest
hospital casualty department immediately.
Side effects:
Like all medicines, Azitrodor® can cause side effects although not everybody gets them.
The most common side effects that occur when taking Azitrodor® are listed below. These may go away during treatment as your body adjusts to the medicine. Tell your doctor if any of these side effects continue to bother you.
- Very common: stomach cramps, feeling sick, diarrhoea, wind.
- Common: dizziness, headache, numbness or pins and needles, being sick, indigestion, loss of appetite, taste disturbance, visual disturbances, deafness, skin rash and /or itching, joint pain, low numbers of lymphocytes , higher number of eosinophils, low blood bicarbonate, tiredness or weakness.
Reporting of side effects:
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed here.
You can also report side effects directly via the Yellow Card Scheme at: www.dorsapharma.com/ (ADR form)
By reporting side effects, you can help provide more information on the safety of this medicine.
Product specification
| Generic name | Azithromycin |
| Trade name | Azitrodor® |
| Dosage form | 250 & 500 mg milky capsules, 100mg / 5 ml white powder for 30 ml suspension and 200 mg /5 ml white powder for 15 and 30 ml suspension |
| Category | Anti infective, Macrolids |
| ATC code | J01FA10 |
| Package | • Box of 1 Alu-PVC blisters of 6 capsules • Box of PVC bottle of 15 and 30 ml suspension |
| Active ingredient | Azithromycin |
| Storage | Below 30°C. Protect from light & moisture. After preparation of suspention, keep it refrigerated and protect from frozen. Do not keep the oral liquid for more than 7 days. |
| Shelf life | 2 years |