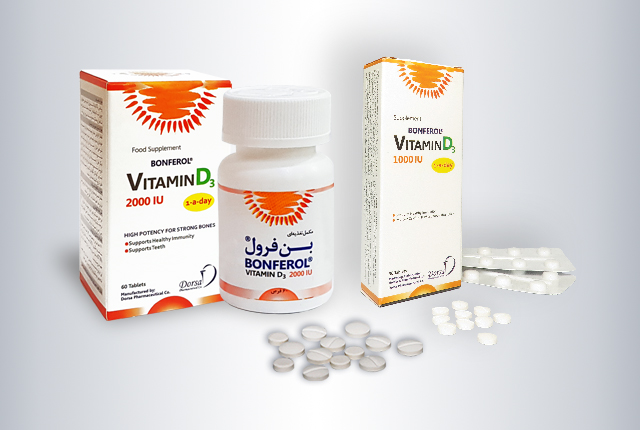
Bonferol®
- Keep out of the reach of children.
- Do not use after the expiry date printed on the label.
- FDA pregnancy category: It is not indicated for use by women.
- Rx only.
- Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use.
Gerenal information
Product specification
| Generic name | Vitamin D3 |
| Trade name | Bonferol® |
| Dosage form | 1000 & 2000 I.U. white round scored film coated tablets |
| Category | Supplements |
| Package | 1000 I.U.:Box of 3 Alu-PVDC blisters of 10 tablets 2000 I.U.: Box of plastic bottle of 60’s tablets |
| Active ingredient | Vitamin D3 |
| Storage | Below 30°C. Protect from light & moisture |
| Shelf life | 2 years |